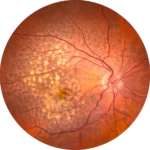
First data showing preliminary evidence of efficacy were presented at the Retinal Cell and Gene Therapy Innovation Summit organized by the Foundation Fighting Blindness
Zürich, Switzerland, May 3, 2024 First data showing preliminary evidence of efficacy were presented at the Retinal Cell and Gene Therapy Innovation Summit organized by the